Which Statement Describes the Valence Electrons in Ionic Bonds
They are shared equally between two atoms. Which statement describes the valence electrons in ionic bonds.
Could Two Atoms Engage In Ionic Bonding Why Or Why Not Quora
Ionic bond also called electrovalent bond type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.

. These valence electrons of the metallic bond is shared amongst many atoms so that the metals can be bounded together. It is a type of chemical bond that generates two oppositely charged ions. A tendency of atoms to react in ways to achieve outer shell of eight valence elections.
Which statement best describes the effect of low ionization energies and low electronegativities on metallic bonding. They are shared unequally between two atoms. They are shared unequally between two atoms.
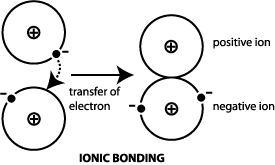
Electrons are mobile within a metal. Ionic bonding is the complete transfer of valence electrons between atoms. An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions.
In ionic bonds the metal loses electrons to become a positively charged cation whereas the nonmetal accepts those electrons to become a negatively charged anion. Which of these is a property of a substance that is composed of atoms that are held together by ionic bonds. How are valence electrons described in a metallic bond.
Which statement describes the difference between an ionic bond and a hydrogen bond. The correct answer is D. During the formation of an ionic bond the valence electrons are transferred from a metal atom to the non-metal atom leading to the formation of an ionic bond.
The question is all about which among the given choices is true or properly describes the valence electron of the metallic bonds. Which statement describes the valence electrons in ionic bonds. Electrons transition from an inner shell to a valence shell.
A In ionic bonds electrons are shared while in covalent bond elections are ripped between two atoms. CH3COOH is a carboxylic acid. Calculate the total number of valence electrons.
In ionic bonds the valence electrons are transferred from one atom to another. The atoms which lose valence electron become positively charged and the one which accepts it becomes negatively charged. The atoms which lose valence electron become positively charged and the one which accepts it becomes negatively charged.
Place a single bond 2 electrons between the central P atom and each H bonded to it. Ionic bonds form when a nonmetal and a metal exchange electrons while covalent bonds form when electrons are shared between two nonmetals. During the formation of an ionic bond the valence electrons are transferred from a metal atom to the non-metal atom leading to the formation of an ionic bond.
October 16 2021 by sarah yalton. The answer to this item is therefore letter the third choice. They are shared equally between.
Add the remaining electrons to complete the octet except H which needs only two match the carbon-carbon bond length to the appropriate hydrocarbon. They are shared among many atoms. Model them as a sea of electrons.
Such a bond forms when the valence outermost electrons of one atom are transferred permanently to. These ionic bonds are very strong in nature. These valence electrons of the metallic bond is shared amongst many atoms so that the metals can be bounded together.
They are transferred from one atom to another. The correct option is A. It conducts electricity when it is dissolved in water.
The valence electrons of atoms in a pure metal can be modeled as a sea of electrons. Therefore the correct option is A. An ionic bond forms when two atoms share valence electrons while a hydrogen bond forms between.
The question is all about which among the given choices is true or properly describes the valence electron of the metallic bonds. During the formation of an ionic bond the valence electrons are transferred from a metal atom to the non-metal atom leading to the formation of an ionic bond. Electrons are transferred from one atom to another.
The correct answer is D. They are transferred from one atom to another. Which statement describes the valence electrons in ionic bonds.
Ionic bond also called electrovalent bond type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonds are formed as a result of complete transfer of electrovalence electrons from one atom to another. In metallic bonds the valence electrons from the s and p orbitals of the interacting metal atoms delocalize.
The atoms which lose valence electron become positively charged and the one which accepts it becomes negatively charged. When one atom gives up one or more electrons from its valence shell to another atom the bond formation occurs. The answer is letter C.
Which statement describes the valence electrons in ionic bonds. They are shared among many atoms. That is to say instead of orbiting their respective metal atoms they form a sea of electrons that.
An aldehyde has an alkyl group with a fromyl group attached to it or CHO. C2H5OH is an alcohol and C6H10O5 is an ether. Part 1 Unit Test 92.
They are shared among many atoms. CH 3 COOH D. They are shared equally between two atoms.
The statement best describes the Ionic and Covalent bonds is ------. Electrons are shared between atoms. These usually occurs between two ions of opposite charges.
An ionic bond forms when electrons are completely transferred between two ions while a hydrogen bond forms between partially positive and partially negative atoms. The atom that donate the electron become a positively charged ion while the atom that received the atom become a negatively charged ion. Delvig 45 The chemical formula that represents an aldehyde is C2H4O.
The answer to this item is therefore letter the third choice. Which statement describes the valence electrons in ionic bonds. C 2 H 5 OH B.
They are shared equally between two atoms. The correct answer is D. Which statement best describes the formation of ionic bonds.
Which statement describes an ionic bond. They are transferred from one atom to another. Such a bond forms when the valence outermost electrons of one atom are transferred permanently to.
They are shared unequally between two atoms.
Out Of Atoms With 3 4 7 And 8 Valence Electrons Which Is The Least Likely To Form A Chemical Bond Quora
Comments
Post a Comment